HI all,
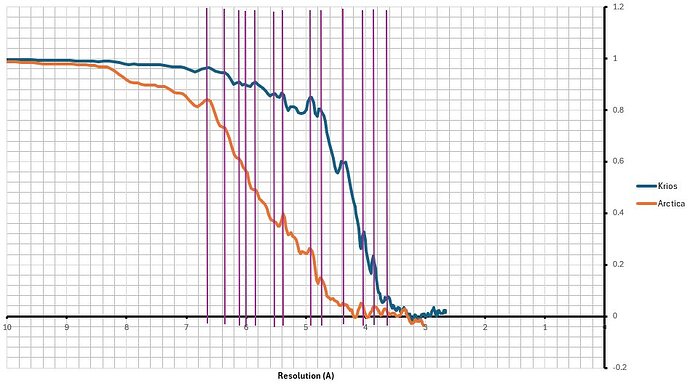
i have a jagged fsc curve, see below. There is a symmetry applied here but even in C1 the peaks and trophs are the same (i just dont see the one below 5A since i dont reach quite the same resolution in C1).
I have another map from a different protein in the same micrographs and the FSC curve looks just fine, no issues at all.
The map looks about right for a 4A resolution, maybe slighly over estimated but nothing major.
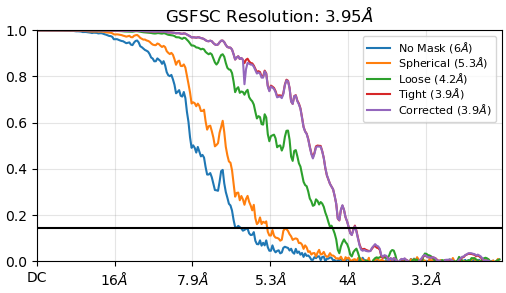
Thoughts?
How many particles? Decent defocus variation across them? How mobile is it? How large is it? How have beam shift groups been treated? Are the mag aniso and beam tilt estimates reasonable?
The sharp drops seem in sync, which makes me think relative particle count is low and defocus spread a little biased?
I’ve seen similar effects before but in very specific samples; lower particle count reconstructions of giant viruses where the capsid has extremely rigid domains and more flexible ones. Medusavirus was a particular problem as it has a variable array of spikes of different types and the larger ones are very flexible (which was somewhat, but not completely, alleviated by block-based reconstruction).
1 Like
Thanks.
There are about 70,000 particles (~10,000 movies … yes not many particles per movies) .The defocus is pretty spread out (-0.5 to -1.6um pretty much continuous), as mentioned an other protein from the same dataset has a FSC that’s just fine. SO i am guessing the issue is not from teh micrographs themselves but more the protein or its mask?? i tried to relax the “dynamic mask far” but that didn’t change much, obviously the curve is slightly different but the peaks are at the same spots.
the particle is very large, it is a donut 500A in diameter and 100A thick. the beam tilt and shift pretty good (it is an AFIS acquisition).
70,0000 particles is too many for that suggestion to be correct I think. If they’re evenly spread across all micrographs that flattens that hypothesis also, although if they’re clustered in a few similar defocus micrographs it might still account for some of it…
Is the other protein which is OK the same sort of shape? If not, do the top views of this one have a large enough hole that defocus refinement (if done) is getting confused by vitreous ice rather than protein?
Were beam shifts clustered? Does the global CTF refinement output look sensible? I avoid doing more than one beam tilt at once (or at all in NU refinement, now), as sometimes they can feedback-loop on themselves and end up with estimating (tens of) thousands of milliradians of beam tilt.
So yes i checked and they are pretty spread out through the defocus gradient. The local defocus refinement doesn’t bring any improvement nor does the refinement of the tilt and trefoil (the number are pretty and consistent with what they should and are on other samples). the FSC is pretty much the same regardless of local/global refinement/symmetry applied/mask relaxation …
yes the beam shift were nicely clustered
the other protein is a helical reconstruction so very different shape and reconstrctuotion strategy (it is my first helical one, so I don’t yet know much about this)
Hm.
As a test, I’d be interested in the results of a symmetry expanded run, recentered to one component with a local refinement (before and after local CTF as well)?
Another thing to try would be Ewald sphere correction (and ESC local CTF), although 500 Å… might not show anything appreciable.
I tried that too  it didn’t change anything.
it didn’t change anything.
what i forgot to mentioned (but also to check and i have just done it) is that i have two other acquisitions (albeit smaller number of movies and particles, about half for one and a tenth for the other) on an Arctica and a Glacios. they both show the same peaks. So overall I am guessing it is due to the protein?
Yup; would seem like there are local correlations at different frequencies, possibly due to mobility of certain region(s) or secondary structure…
could this be due to the high symmetry? the protein is a C32 (with a pseudo D32)
Maybe but I’d be a bit surprised even so. That said I’ve not worked on anything with C32 symmetry (highest I’ve played with was C18…)