Hi everyone,
I’ve got a soluble protein complex (45kDa+55kDa) for cryo-EM study.
Movies: 0.58A, 300V, 40e/A2; Particle Extraction: 256pix->128pix
1st 2D Classification
1st Select 2D:get rid of most dummy particles (7,695,260 down to 1,123,196)
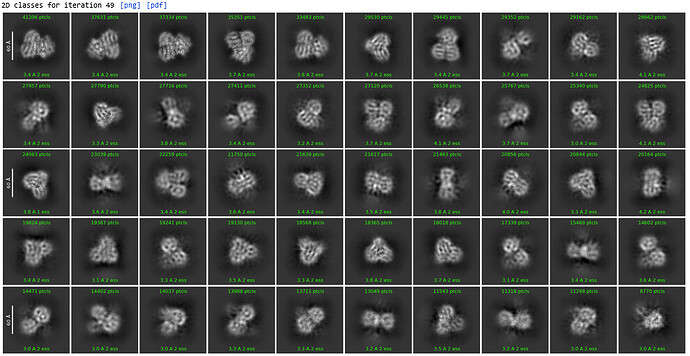
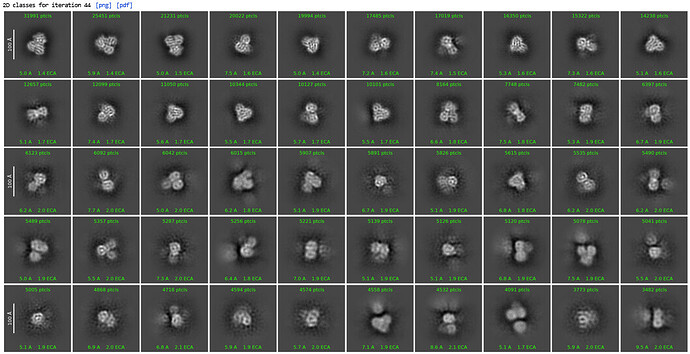
2nd 2D Classification, same settings
Could see secondary structures such as a-helices.
2nd Select 2D: (1,123,196 down to 350,114)
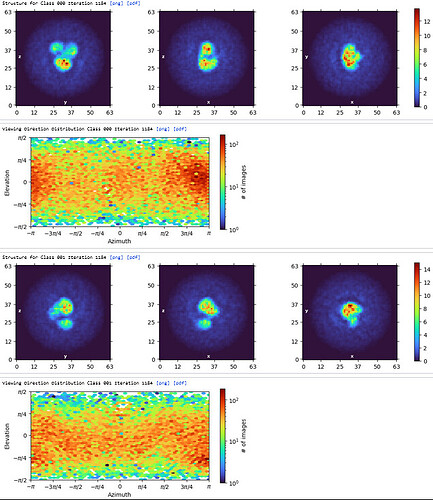
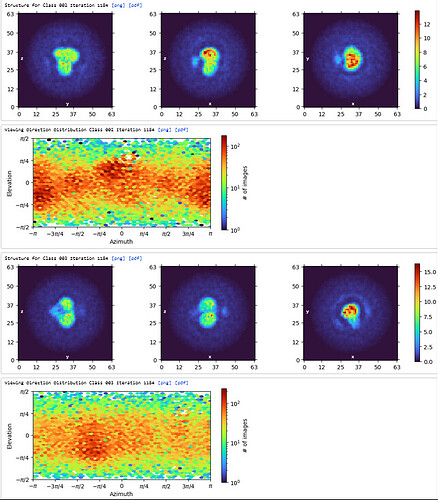
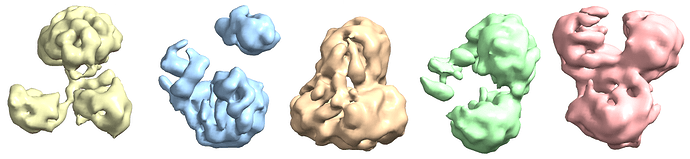
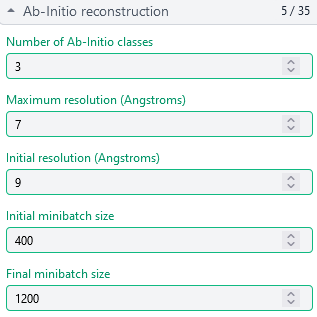
Ab-Initio (3 classes; Particles: 2nd select 2D, 350,114)
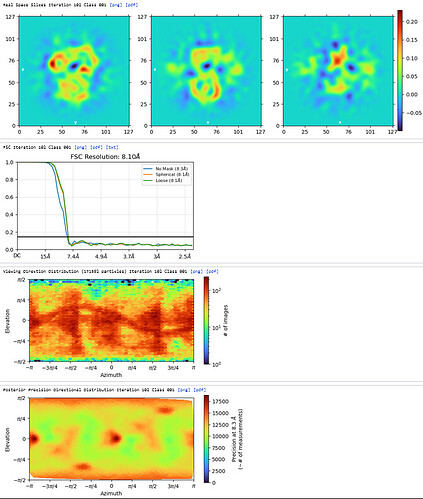
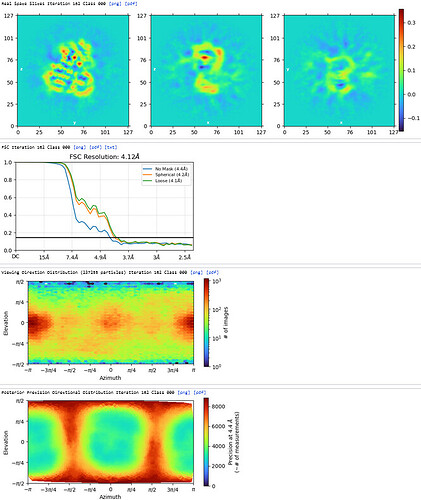
1st Hetero Refinement (Volume: classes0&1 from Ab-Initio job; Particles: 1st select 2D, 1,123,196; Force hard classification)
2nd Hetero Refinement (Volume: classes0&1 from Ab-Initio job; Particles: class0 from 1st Hetero Refinement, 608,026; Force hard classification)
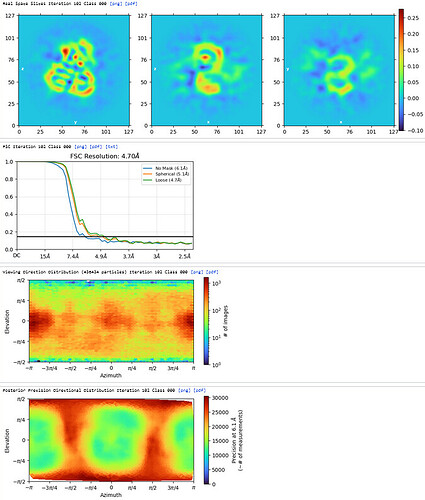
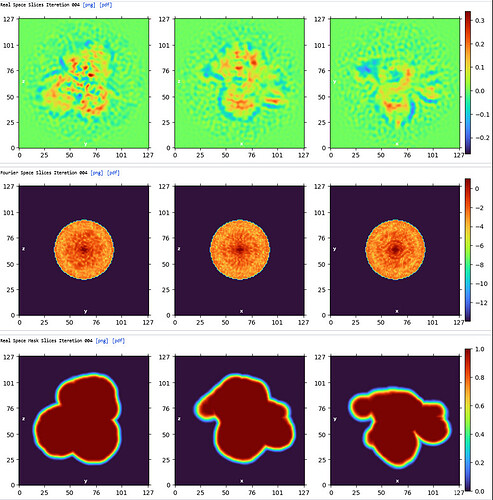
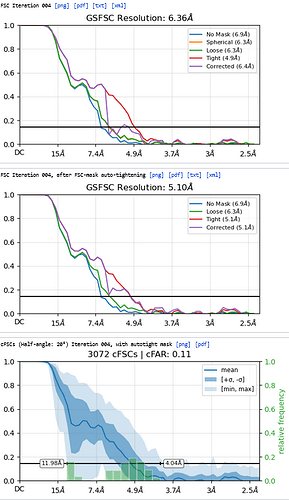
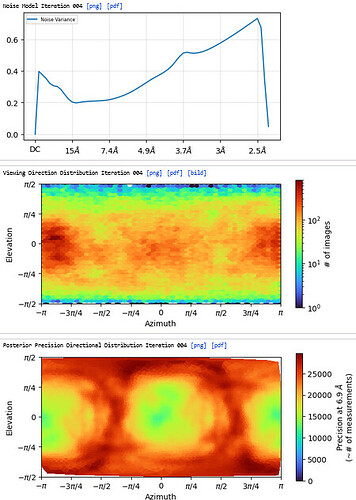
3rd Hetero Refinement (Volume X2: class0 from 2nd Hetero Refinement; Particles: class0 from 2nd Hetero Refinement, 436,434)
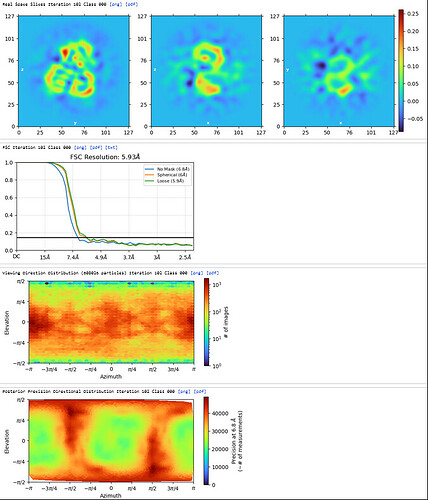
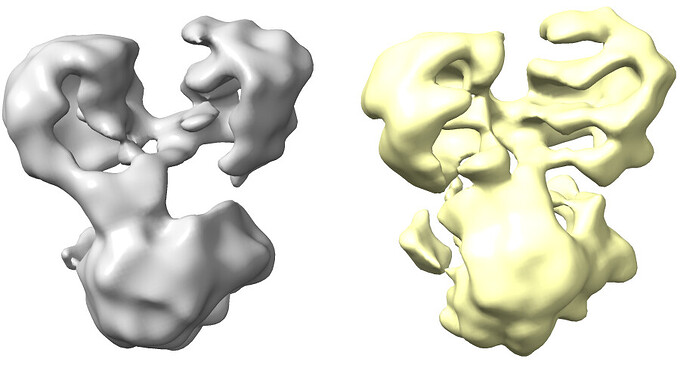
The resolution of class0 could get to 4.1A.
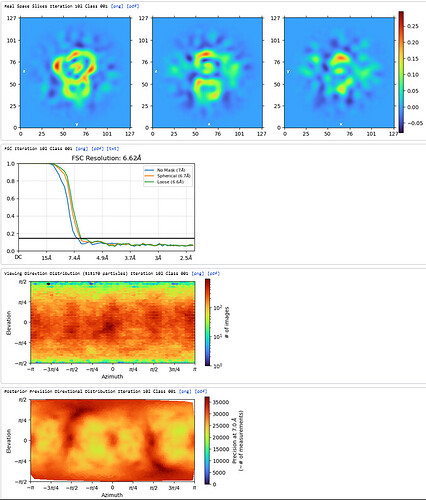
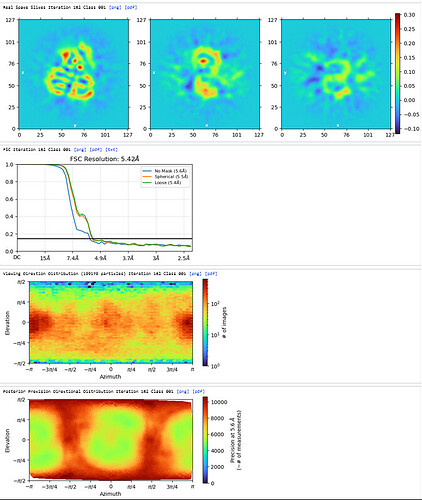
Homo Refine (Volume&Particles: classe0 from 3rd Hetero Refinement, 237,255; Minimize over per-particle scale)
The resolution got worse, even worse than 3rd Hetero Refinement.
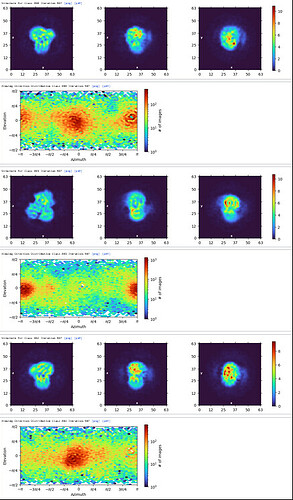
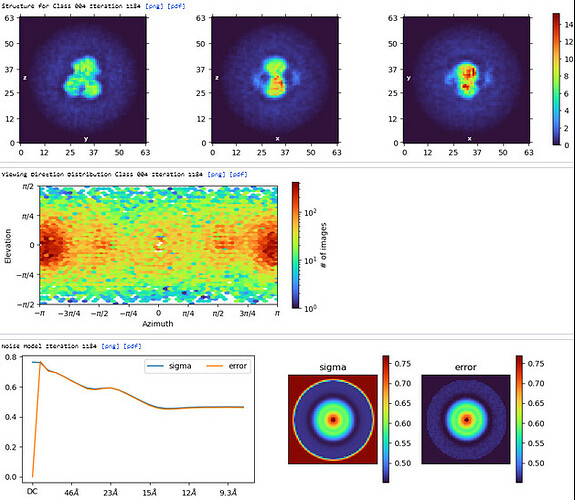
The maps are shown below:
_______________Homo Refine_________________________classe0 from 3rd Hetero Refine
Is there anything I could try to get a better 3D reconstruction?